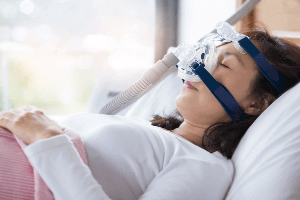
 Philips Respironics is recalling millions of its CPAP and BiPAP machines because they have been linked to a higher risk of developing lung cancer and other severe health issues. The recalled devices are used to treat sleep apnea and they were all made before April 26, 2021.
Philips Respironics is recalling millions of its CPAP and BiPAP machines because they have been linked to a higher risk of developing lung cancer and other severe health issues. The recalled devices are used to treat sleep apnea and they were all made before April 26, 2021.
Sleep apnea is a potentially serious sleep disorder in which a person’s breathing abruptly stops and starts. The disorder is commonly treated using a Continuous Positive Airway Pressure (CPAP) machine that helps the wearer breathe more easily during sleep.
If you used any of the recalled Philips products to treat your sleep apnea and later developed lung cancer or other health issues, you may be eligible for compensation.
Our class action attorneys are prepared to help you pursue the compensation you need for medical bills, lost wages and other damages associated with your injuries.
The consultation is free and there are no fees unless we win.
Why Did Philips Issue the Recall?
According to the recall notice to consumers from Philips, the company was made aware of a potential issue with the sound abatement foam used in several of its CPAP and Bi-Level Positive Airway Pressure (BiPAP) machines. The foam used is meant to reduce noise while the machines are in use by the wearer since they are meant to be used while a person sleeps.
The problem with the foam is that as it degrades, particles of it begin to break away and find their way into the machines’ air pathways, where the particles may be absorbed or ingested by the wearer. The foam is made up of polyester-based polyurethane which, if inhaled, poses potentially serious health risks, including the increased risk of cancer and pulmonary fibrosis.
Black debris from the foam has caused users to experience various symptoms, such as:
- Headaches
- Vomiting
- Asthma
- Nausea
- Hypersensitivity
- Skin irritation
- Inflammation
- Dizziness
Philips claims that the causes of the degradation of the foam are unapproved cleaning methods, exposure to high heat and high humidity environments. However, it is not yet clear whether Philips took the necessary precautions to keep the foam from deteriorating.
What Products Are Affected?
The recall was only issued to U.S. consumers, so the affected products are only those sold in the U.S. that were manufactured prior to April 26, 2021. The affected products include the following:
CPAP and BiLevel PAP Devices
Continuous Ventilator, Minimum Ventilatory Support, Facility Use –
- E30 (Emergency Use Authorization)
Continuous Ventilator, Non-life supporting –
- DreamStation ASV
- DreamStation ST, AVAPS
- SystemOne ASV4
- C-Series ASV
- C-Series S/T and AVAPS
- OmniLab Advanced+
Noncontinuous Ventilator –
- SystemOne (Q-Series)
- DreamStation
- DreamStation Go
- Dorma 400
- Dorma 500
- REMstar SE Auto
Mechanical Ventilators
Continuous Ventilator
- Trilogy 100
- Trilogy 200
- Garbin Plus, Aeris, LifeVent
Continuous Ventilator, Minimum ventilatory support, facility use –
- A-Series BiPAP Hybrid A30
- A-Series BiPAP V30 Auto
Continuous ventilator, non-life supporting –
- A-Series BiPAP A40
- A-Series BiPAP A30
Anyone who used one of these defective products may be at risk of developing serious health risks. It is important to note that healthcare providers and sleep laboratories were also using these devices on patients and participants of sleep studies.
What is the Response from the U.S. Food and Drug Administration (FDA)?
Once the FDA became aware of the recall from Philips, the federal agency issued its own notice to consumers and classified the recall as a Class I, which is the most serious type of recall.
The FDA urges consumers to discontinue the use of the defective devices and work with a medical professional to determine an appropriate option for continued treatment for sleep apnea.
Have Any Lawsuits Been Filed?
Soon after Philips issued the recall, a class-action lawsuit was filed on behalf of consumers who were harmed by the CPAP machines and ventilators. The complaint claims that Philips knew about the potential risks well before they issued a recall since they had received complaints about black particles in their machines for many years.
Additionally, the lawsuit alleges that the only reason Philips issued a recall is because it is releasing its next generation of CPAP products that do not have the same problems.
Have You Used One of the Recalled Machines? Call Us Today
If you used any of the recalled Philips CPAP machines and ventilators and then experienced adverse side effects, you may be able to file a claim for compensation.
Our experienced attorneys are prepared to discuss the facts of your claim during a free consultation to see what your legal options may be for recovering compensation for medical bills and other damages.
Call us today (574) 444-0741 to schedule your free consultation.











